
Elements of an Efficient Fertility Program
Natural Sources of Nitrogen
- Posted: Dec 18, 2023
- Author: Nature Safe Fertilizers
- Nutrients, Innovation
Crop fertility requires a compatible relationship between the atmosphere, plants, and soil. Multiple other factors enter into the relationship. Some, like rainfall or humidity, cannot be modified. However, growers can manipulate many other factors for profitable production. One of the critical factors we can tweak is nitrogen.
Even though nitrogen is one of the most abundant elements on earth, nitrogen deficiency is the most common nutritional problem affecting crops. This deficiency is because nitrogen from the earth's crust and the atmosphere is not directly available to plants.
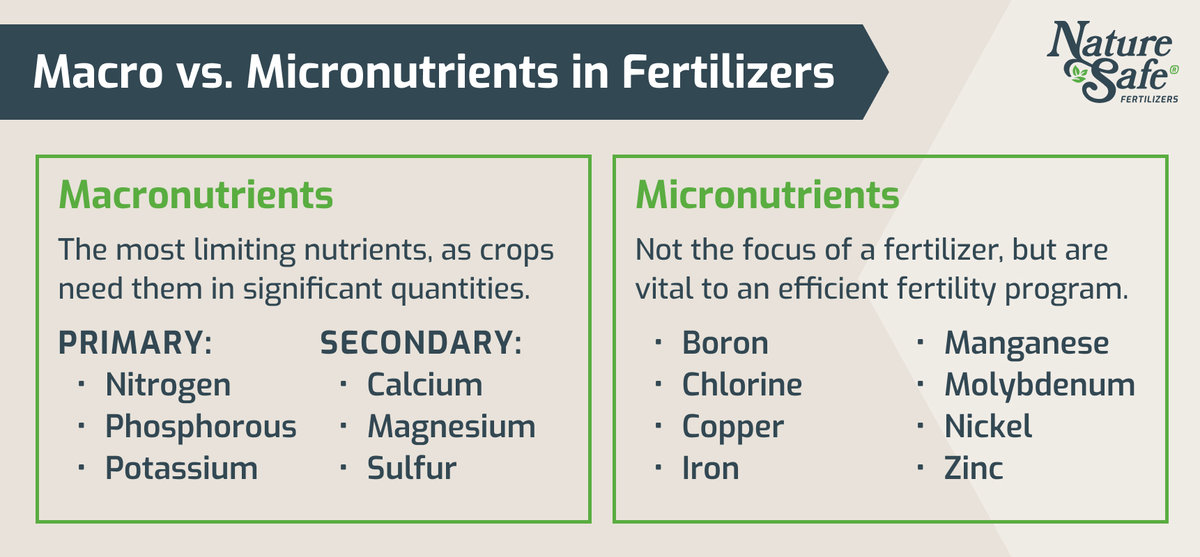
Macro vs. Micronutrients
Crops need an incredible variety of nutrients to grow properly and productively, some in more abundance than others. Most commonly used agricultural fertilizers contain the three macronutrients: nitrogen, phosphorus, and potassium. Nature Safe fertilizers will also contain micronutrients present in the plant and animal products that they’re made from.
Fertilizers can include materials such as:
- Virgin raw material (previously unused copper, zinc, etc.)
- Compost and recycled organic matter (feather, blood, and bone meal)
- Wastes (sewage sludge and industrial waste)
Chemical and synthetic fertilizers feed crops directly. However, they lead to soil depletion and contaminate ground and surface water over time. Organic fertilizers, like Nature Safe’s, provide the soil with long-term nutrients and trace minerals. The combination of macro and micronutrients sustains plant and soil health.

Macronutrients
Macronutrients are considered the most limiting nutrients, as crops need them in significant quantities. The vital macronutrients needed by plants are:
- Nitrogen: helps in chlorophyll production, impacts leaf development, and strengthens foliage
- Phosphorous: supports root and flower growth and helps plants survive environmental stressors and difficult climates
- Potassium: contributes to early plant growth and assists in water retention, strengthens and aids plants in disease and insect resistance
- Calcium: enables growth and development of cell walls, resists diseases, assists in cell metabolism and nitrate uptake
- Magnesium: contributes to plant health and color
- Sulfur: resists disease, aids in the production of amino acids/proteins/enzymes/vitamins, helps to form and grow seeds
Macronutrients will break down into primary and secondary—the “big three” (nitrogen, phosphorus, and potassium) are considered primary, since these are required in the highest quantities. Other macronutrients are considered secondary.
Micronutrients
Micronutrients aren’t usually the focus of a fertilizer, but that doesn’t mean they aren’t a vital part of an efficient fertility program. The important micronutrients are:
- Boron: assists in pollination and fertilization, regulates metabolism, and is critical for new growth
- Chlorine: essential for photosynthesis and root growth
- Copper: activates plant enzymes
- Iron: enables the formation of chlorophyll
- Manganese: assists in chlorophyll formation and activates enzymes in the growth process
- Molybdenum: helps transform nitrate nitrogen into amino acids
- Nickel: aids in life cycle completion and viable seed
- Zinc: acts as a regulator and is essential in plant and root growth
While macronutrients aren’t needed in nearly the same quantities as macronutrients, a deficit of any of them can mean an unproductive crop, and less money in your pocket.
Nitrogen is a Macro, Primary Ingredient
Plants comprise 3-4% nitrogen in above-ground tissue, much higher than any other nutrient. Nitrogen, in turn, is directly responsible for plant development, growth, and reproduction.
Nitrogen plays a fundamental role in energy metabolism and protein synthesis, which are directly related to plant growth. It promotes cellular multiplication, photosynthesis activity, and chlorophyll formation.
Above all, nitrogen involves itself in the aerial zone—the above-ground part of a plant that we see. Plants with nitrogen deficiency show a loss of color and vigor. Their growth slows, and their leaves will fall off, starting at the base of the plant.
How Do Crops Utilize Nitrogen?
Plants utilize nitrogen in the forms of nitrate, nitrite, and urea, which they absorb from the soil through their root hairs. Then, they reduce them into ammonium ions for use in amino acids, nucleic acids, and chlorophyll.
While we recognize nitrogen is essential to your crops, it can sometimes take time to understand its specific role and ensure it's available in optimal amounts for production. So, to simplify, crops utilize nitrogen in many ways, but the most important are the following:
- Amino acids: building blocks of plant proteins, develops vital plant tissues and cells
- Chlorophyll: allows for utilization of the sun's energy and ensures energy is available to the plant when needed
- Nucleic acid: forms DNA, transfers desirable crop characteristics that aid in plant survival, and holds genetic code within the plant nucleus
- Photosynthesis: high rates of carbohydrate formation and vigorous growth and development
Natural Sources of Nitrogen
Nitrogen goes through many transformations in the soil, called "the nitrogen cycle." It’s a repeating process where nitrogen moves through living and nonliving things—the atmosphere, water, soil, plants, animals, and microorganisms.
The three steps of the nitrogen cycle are:
- Fixation
- Mineralization
- Nitrification
Nitrogen has to change forms to move through the different processes. In the atmosphere, it exists as a gas (N2). However, in the soil, it changes to nitrogen oxide (NO) or nitrogen dioxide (NO2). When used as a fertilizer, nitrogen occurs in the form of ammonia (NH3) or ammonium nitrate (NH4NO3).
Leaching occurs when a mineral or chemical form of nitrogen dissolves in water and leaks out of the soil, potentially polluting waterways. Excessive leaching can also flush essential macro and micronutrients from the ground, causing crop deficiencies.

The Atmosphere
The first stage of the nitrogen cycle, fixation, moves nitrogen from the atmosphere into the soil. Our atmosphere contains a large amount of nitrogen gas (N2), but nitrogen in gas form is unavailable to plants.
Fixation converts atmospheric nitrogen into a form that plants can absorb through their roots. Lightning provides the energy for nitrogen to react with oxygen and create nitrogen oxide (NO) and nitrogen dioxide (NO2), which later enter soils through rain or snow.
Nitrogen fixation can also occur through the industrial processes that create fertilizer. By employing high heat and pressure, professionals can combine atmospheric nitrogen and hydrogen to form ammonia (NH3) and process it further to develop ammonium nitrate (NH4NO3), which can be added to soils and utilized by plants.
Organic Matter in Soil
The second stage, mineralization, takes place in the soil. Here, Nitrogen moves from organic materials (manure or decomposing material) to an inorganic nitrogen form that plants can use. Microbes act on the organic material, releasing its nutrients into the soil via ammonia.
The third stage, nitrification, also involves organic matter and soil. During this stage, the ammonia produced during mineralization converts into nitrites and nitrates with the help of bacteria. This process creates an extra stash of available nitrogen that plants can absorb as needed through their roots.
Other Sources of Nitrogen
Farmers need to provide enough nitrogen for crops to maintain good yields, product quality, and profitability. However, traditional methods can be costly and could be more consistent in long-term soil fertility and health. Organic farming emphasizes rotation with cover crops and organic fertilizers.
Cover Crops
Farmers use cover crops to add or recycle nitrogen into the soil. Legumes are most often associated with nitrogen addition because they have developed symbiotic relationships with soil bacteria. These bacteria convert nitrogen gas in the air (the form plants cannot use) to ammonia (the form plants can use).
Non-legumes, such as brassicas and grasses, take nitrogen from the soil and prevent it from leaching. When these crops are killed and allowed to decompose, nitrogen is released back into the ground to feed the next crop.

Organic Fertilizer
High-quality organic fertilizers offer natural decomposition, making them easy for plants to digest. Organic fertilizers feed the soil with nutrient-rich sources of organic matter that break down slowly to sustain plants. They include animal waste and byproducts, such as bat and bird guano, fish and kelp fertilizers, and feather, bone, and blood meals.
Organic fertilizers work alongside regenerative agriculture, allowing your crops to be self-sustaining. Instead of depending on you for a supply of synthetic fertilizers, plants can draw their nutrients from a healthy soil environment--resulting in high-yield crops and beautiful flowers. Plus, the gentle nature of organic fertilizers means you don't have to worry about them burning plant roots or foliage as a chemical fertilizer would.
High-Nitrogen, Nature Safe Fertilizers
Nature Safe's 13-0-0 blending base is the highest amount of organic listed nitrogen available today. You can find this ingredient in many other Nature Safe fertilizers, such as 10-2-8 and 27-0-2. This specialized blending product is OMRI Listed® and is allowed under NOP guidelines, validating its use in certified organic crops.
Research from Clemson University shows that Nature Safe 10-2-8 delivers double the 100% organic NPK value. With 11 times more than the competitor's amino acid content, it provides food value at half the cost.
Nature Safe as Part of an Efficient Fertility Program
Nature Safe backs its products with decades of scientific research. Our fertilizer is rich in high-quality nutrients and provides slow availability in a 10 to 12-week release period. Pound for pound, we offer more nutrition in a single bag than any other organic fertilizer on the market.
We're the only organic fertilizer company to control and source our ingredients from beginning to end, providing you with a reliable and consistent product. Reach out to us today to see which of our products best suits your needs and could save you money in the long haul.
Thank you for reading our blog! Sign up and we’ll email the next one directly to you.
